Abstract
Introduction
The presence of deletion 17p or monosomy 17 (del(17p)/-17) detected by fluorescence in situ hybridization (FISH) is well-established as a high-risk (HR) factor in multiple myeloma (MM). The del(17p)/-17 clone size and the clinical impact in patients that have ≥60% involved nuclei is controversial. Optimal treatment is also controversial, with tandem autologous stem cell transplantation (ASCT) explored but not uniformly adopted in the real world (ENM02, StaMMina). At our center, we have routinely offer tandem transplantation for del(17p)/-17 and now review our experience over a 10-year period, including the significance of del(17p)/-17 genetic variations on outcomes.
Methods
We performed a retrospective chart review of all patients identified with del(17p)/-17 patients who were offered tandem transplant and underwent at least one ASCT at Princess Margaret Cancer Centre from 2009-2019. Patient and disease characteristics, responses, and survival outcomes were collected from the Myeloma and Transplant databases and electronic patient records under REB approval.
Results
Patient, disease, and treatment characteristics.
We identified 100 patients with del(17p)/-17 who underwent ASCT at our center. These patients were separated into three groups: those with a deletion of the TP53 locus (57%), those that had monosomy 14 (17%), and those with a relative loss the TP53 locus (eg. aneusomy or polyploidy,13%). The median % of nuclei with del(17p) was 39% (range 5-93%), with 27 (25%) over 60%. 28% of patients carried at least one other cytogenetic abnormality besides del(17p), most commonly t(4;14). All patients were intended for tandem transplant, but only 69 (69%) completed both transplants. Reasons for not proceeding to a second transplant were: patient declined (29%), toxicity (26%), clinical trial (19%), progression prior to the second transplant (13%), with one early death (3%). Median age at transplant was 61 years (range 40-72); most were male (61%) with advanced R-ISS II and III (97%).
The most common induction regimen used was cyclophosphamide, bortezomib and dexamethasone (CYBORD)(87%), with 10% requiring a 2 nd induction regimen for inadequate response/progression. Standard conditioning was melphalan 200mg/m 2 for both first and second transplants. Median time from diagnosis to first ASCT was 5.9 months (range 3-24) and time from first to second transplant was 3.3 months (range 1-7). Most patients (90; 84%) received maintenance therapy post-transplant: 47% lenalidomide, 29% multiple agents (commonly lenalidomide and a proteasome inhibitor)
Response and survival outcomes
After induction, the rate of VGPR (very good partial response) or greater was 53%, increasing to 73% after 1 st ASCT, 85% after 2 nd ASCT. At a median follow-up of 32 months (range 3-130), the median PFS for all patients was 39.2 months 95% CI (24.1-46) and median OS was not yet reached (NYR) (95% CI 57.9-NYR). When analyzed by whether single or tandem transplant was performed, median PFS was 21.8 months (95% CI 10.3-43.3) for single ASCT, and 42.1 months (95% CI 32.8-NYR) for tandem ASCT (P=0.0096). The median OS was 57.6 months for single ASCT (95% CI 22.2-86.5), but NYR for tandem ASCT (95% CI 105.6-NYR) (p=0.0022).
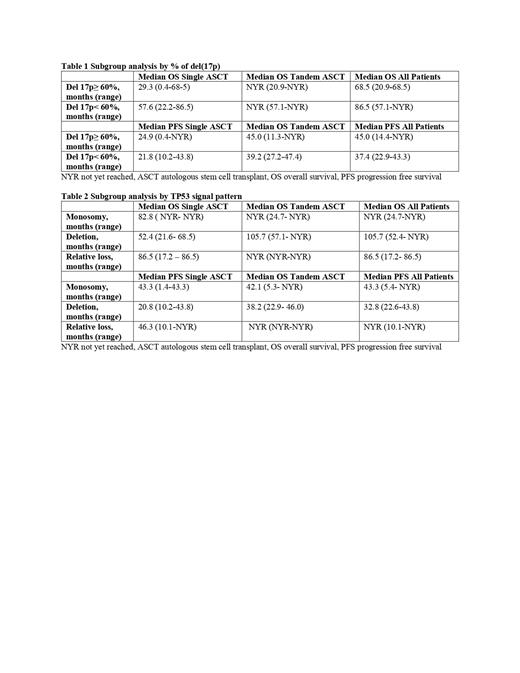
On subgroup analysis, patients with ≥60% del(17p) achieved a median PFS 45 months (95% CI 14.4-NYR) and median OS 68.5 months (95% CI 20.9-68.5), median was only PFS 24.9 months (95% CI0.4-NYR) and median OS 29.3 months (95% CI 0.4-68.5) for those who received single ASCT. (table1)
TP53 signal pattern analysis (table 2) showed the median OS for patients with monosomy 17 was NYR, while it was 105.7 months for patients with deletion, and 86.5 months for those with relative loss. Median PFS was 43.3 months for the monosomy group, 32.8 months for deletion group, and NYR for relative loss.
Conclusions
Our experience shows that MM patients with del(17p)/-17 appear to have deeper responses and improved outcomes with more aggressive tandem transplantation. However, approximately 1/3 of our patients intended for tandem transplant did not undergo a second transplant, identifying a focus of future investigation and optimization. Our analysis also suggests that clone size of del(17p) >60% does impact OS negatively, but improved with tandem transplantation. The type of deletion also appears to affect outcomes with deletion having worse PFS but not worse OS.
Prica: Astra-Zeneca: Honoraria; Kite Gilead: Honoraria. Reece: Takeda: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Millennium: Research Funding; Sanofi: Honoraria; BMS: Honoraria, Research Funding; Karyopharm: Consultancy, Research Funding; GSK: Honoraria. Trudel: Amgen: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; GlaxoSmithKline: Consultancy, Honoraria, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Genentech: Research Funding; Roche: Consultancy; Sanofi: Honoraria; Pfizer: Honoraria, Research Funding. Chen: Beigene: Membership on an entity's Board of Directors or advisory committees; Astrazeneca: Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy.